Inguinal plugs are made from polypropylene material. As polypropylene oxidizes it becomes more rigid and brittle, which often leads to erosion. These plugs utilize more polypropylene material per unit area of defect than flat mesh, which means they are subject to faster oxidation and more inflammation. This along with their three-dimensional plug shape promotes contracture and migration.
Polypropylene is made up of both Crystalline segments, which are rigid, and Amorphous segments which are flexible. The Amorphous segments are more susceptible to oxidation and are the first to erode. Over time this process results in only the Crystalline segments remaining and the mesh loses its flexibility. When this happens, the mesh becomes harder than the surrounding tissues and with movement the tissue gives way to the mesh. This process causes the mesh implant to erode and migrate.
Three different manufacturers have popular polypropylene plug designs: Bard, Ethicon and Atrium. Bard makes the Prefix Plug, Ethicon makes a plug that is part of the Prolene Hernia Mesh System, and Atrium makes the Proloop plug. All of these designs are made from this potentially harmful polypropylene material.
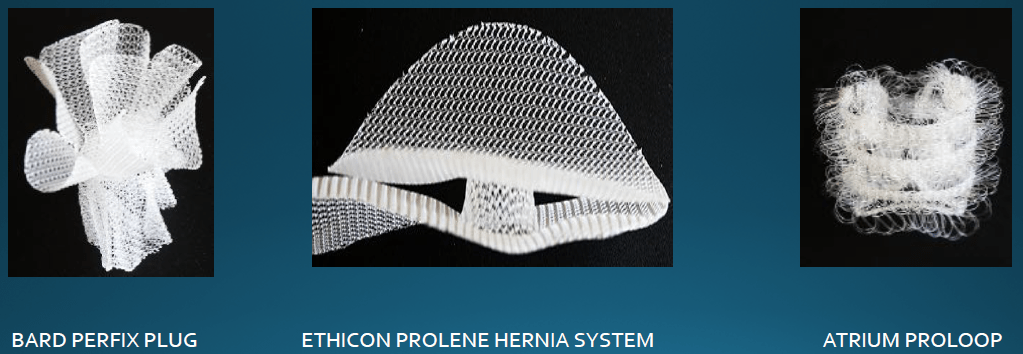
Two specific design factors contribute to an increased inflammatory response; the amount of polypropylene material in these implants and their shape. When human tissue encounters a foreign body it often causes an inflammatory response. The increased volume of polypropylene material contained in these implants often results in more inflammation. Also the three dimensional shape of these implants exposes a larger surface area of tissue to the polypropylene material. When this type of inflammatory response occurs it creates fibrin tissue and results in the formation of scar tissue. When fibrinous tissue bridges the pores of the mesh the filaments are pulled together and cause the mesh to contract.
Composite Ventral patches were designed specifically for the purpose of ease of implantation; not safety and effectiveness. The unique design aspects of these patches make them easier to implant in the hands of less skilled surgeons. They are designed to be intraperitoneal allowing them to be placed next to the bowel and alleviating the need to access one of the extraperitoneal planes. Also, the flexible internal rings eliminate the need to painstakingly assure the mesh is flat and flush with the abdominal wall and properly tensioned.
In order to facilitate the ease of placement with these patches they contain adhesion barriers. Adhesion barriers are a resorbable layer separating the mesh from the tissue. Different manufacturers use different adhesion barriers. For instance, the Atrium C-Qur mesh product uses fish oil, Ethicon’s Proceed product utilizes cellulose and Bard’s Ventralex ST uses hyaluronic acid. All of these constitute additional foreign material that can cause inflammation. When the resorbable layer goes away it leaves the bowel in direct contact with the polypropylene material while in the presence of augmented inflammation. This can result in adhesions of the polypropylene to the bowel resulting in entrapment, perforation, fistula and recurrence. The designs of these plugs and the patches are causing many of them to fail prematurely and resulting in an increased rate of complications, often leading to additional surgeries.
If you or a loved one have suffered from pain, infection, organ damage or hernia reoccurrence leading to subsequent surgery you may be entitled to compensation. Please contact us today.
There are time limits by which you need to file a product liability lawsuit. Those limits could time bar your case if it is not filed in a timely manner.
Complete the Free Case Review form and one of our attorneys will reach out to you within 24 hours to conduct a free and confidential evaluation of your case.